Workshop
Filter By
Display 73 - 81 from 128
Requirements for Clinical Trials of Medical Devices
Brief: Explain the requirements for conduct clinical trials in Saudi Arabia according to ISO 14155 Work ...
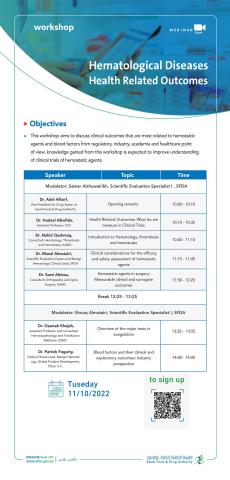
Non-Interventional (Observational) Studies in Post-Marketed Drugs Safety
• Non-Interventional (observational) studies design and quality conduct • Methodological quality (risk of bias) asse ...
Introduction to Medical Device Executive Regulation and Medical Device Requirements U ...
Work Shop Link Remotely : Click Here
Overview on the new SFDA IT service "Overseas Medical Devices Manufacturer Account"
Brief: In this workshop, attendance will learn the following points regarding the new service of “Overseas Medical De ...
Medical Devices Marketing Authorization (MDMA) Requirements for contact lenses and co ...
Brief: Contact lenses' types and contact lenses solutions Overview about the relevant technical ...
Product Sterilization Requirements for Medical Devices
Brief: - Overview of Sterilization requirements - Relevant standards - Advice & recommendations ...
Demonstration of the Medical Device post market Requirements
Brief: • Corrective action requirements for safety alerts of medical devices • Reporting and investigation require ...
Clinical Trials of Medical Device Requirements
Brief: Explain the requirements for conduct clinical trials in Saudi Arabia according to ISO 14155 Work ...